Iobit driver booster 40 pro serial key keygen
25 comments
The best cryptocurrency exchange site
Cancer treatment modalities using macroscopic drug delivery methods like chemotherapy, thermal-therapy and to an extent radiotherapy have had high cure rates but are often accompanied by severe side effects.
For instance Chemotherapy uses chemicals, which selectively kill rapidly diving cells. Although a lot of varieties of cancer exhibit this feature, healthy cells like in hair, bone marrow and digestive tract, are also fatally affected.
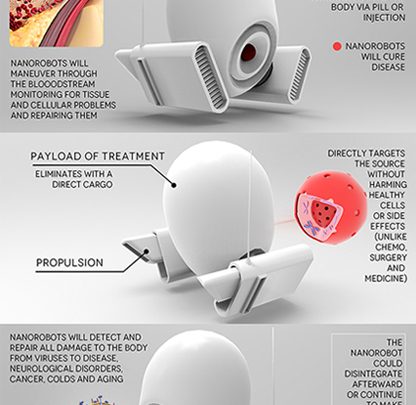
Recent breakthrough in targeted treatment for deadly diseases have led to the invention of molecular machines, called Nanorobots. Introduction of Nanorobots in controlled and targeted drug delivery has revolutionized medicine with special interest in cancer diagnosis and treatment.
Nanorobots, unlike their macro or even milli counterparts are not actually machines with moving parts and conventional control systems with microprocessors. Rather these are made with organic material and intelligence is programmed into the units via genetic modification of their building blocks.
Most nanorobots structurally consist of a sensing agent and a payload. The sensing agent could be one or more chemical strands, each of which are activated by a distinct feature in the targeted cell.
Similar to AND logic gates, when all the sensing agents are activated, the nanobot delivers the payload to the targeted cell. Further, the payload could consist of a drug or a fluorescent tag, depending on the application, viz.
Since these molecular delivery trucks can be programmed to release their payload only when the targeted cell is in correct diseased state, they achieve a specificity that few other treatment techniques can match.
Several classes of nanorobots have been invented lately. These devices were made of a series of DNA strands linked in 2D chains and thereby folded into 3D structures, that could selectively open and close.
Each bot carries two molecular payloads: The sensing agent is called an Aptamer, a molecule that recognizes the surface of a particular cancer cell. Upon recognition, the container dissolves, and payload penetrates the diseased cell initiating a self-destruct sequence in the cell. Since identification of a sensing agent that activates for a single cell type is an arduous task, Rudchenko et al [ 2 ] improved upon the concept by using multiple simple molecules chained to form a robot.
These were designed to seek a specific set of blood cells and tag them. This would in principle also allow for treatment and killing of specific cells. The advantage of this technique is identification of cells that do not possess a single distinctive feature.
Since the surface receptor or sensing agent is made up of multiple DNA strands, it spares similar healthy cells. On cells where all components receptors are attached, the robot is functional and results in tagging of the cell. A similar breakthrough was made by Park et al [ 3 ] concurrently in Korea called Bacteriobot, where non-toxic bacteria, salmonella in this case, are genetically modified to attract chemicals released by cancer cells. These nanobots actively seek cancer cells and deliver drugs to them.
The bacteria are engineered to have receptors, which bind to biochemicals secreted by diseased tissue; thus allowing the bacteria to diagnose cancer, and move towards the cell using flagella. Currently this technique is limited to detecting solid cancers like in breast or colorectal tumors, but it has potential to treat other tumors as well. Nanorobots have been shown to have high potential in-vitro experiments. Next steps would be scaling these devices for in-vivo testing in mice and human tissue.
In-vitro testing in petri dishes already required billion devices, which would scale to several trillions for these to be feasible cancer treatment modalities.
Nonetheless the positive results in nanorobots have brought in new hope to cancer research. Nanorobots in Cancer Treatment Cancer treatment modalities using macroscopic drug delivery methods like chemotherapy, thermal-therapy and to an extent radiotherapy have had high cure rates but are often accompanied by severe side effects. References [1] Douglas, Shawn M. Template adapted from Sergey Karayev.